X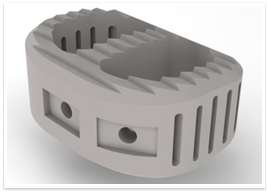 iphos™ ALIF, a VESTAKEEP® PEEK-based (polyetheretherketone) spinal implant developed by DiFusion Technologies, has received U.S. FDA 510(k) approval for use in Interbody Fusion Devices (IBF) devices.
iphos™ ALIF, a VESTAKEEP® PEEK-based (polyetheretherketone) spinal implant developed by DiFusion Technologies, has received U.S. FDA 510(k) approval for use in Interbody Fusion Devices (IBF) devices.
Background
DiFusion Technologies claims VESTAKEEP® PEEK is characterized by its superior biocompatibility and biostability making it suitable as a raw material used in the development of medical devices. Its excellent sterilization resistance and good combination of stiffness and ductility make it excellent for use in spinal implants that must meet extremely high mechanical, thermal, and chemical requirements.
Xiphos ALIF is an anatomically correct shape to improve spinal alignment and height restoration. It’s open structure permits bony fusion throughout the implant itself and its unidirectional teeth provide stability at the operative motion segment and prevent dis-lodgement.
Company comments
“DiFusion Technologies is very excited about the FDA 510(k) approval of the VESTAKEEP® PEEK-based Xiphos™ ALIF spinal implant,” said Derrick Johns, managing director of DiFusion Technologies. “This milestone helps pave the way for more innovative medical devices developed from bioactive polymers. Evonik’s VESTAKEEP® PEEK’s strength and ductility proved to be critical for the FDA approval and the excellent test results Xiphos™ ALIF received.”
“We are pleased with the approval DiFusion received using VESTAKEEP®,” said Sanjeev Taneja, vice president of Evonik’s High Temperature Polymers Business. “Evonik always strives to meet the needs of its customers and helping DiFusion Technologies achieve FDA 510(k) approval for Xiphos™ ALIF is an example of our efforts. This marks the second product line DiFusion is using VESTAKEEP® PEEK for and demonstrates the strong relationships necessary for success in this space.”
Source: DiFusion Technologies





