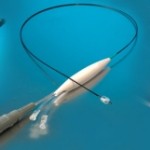
Based in Eindhoven, Netherlands, Xeltis is a clinical-stage developer of advanced polymer-based restorative cardiovascular devices. The company has now announced … continue reading “First-Ever Pivotal Trial of Synthetic Restorative Pulmonary Heart Valve”
Products
Pain Relief Boost with Spinal Cord Stimulator Approval
Pain relief using implantable neurostimulation has come on a lot in recent times. For evidence, try searching our pages. Now … continue reading “Pain Relief Boost with Spinal Cord Stimulator Approval”
U.S. FDA Approves Pipeline™ Flex Embolization Device with Shield Technology™
Medtronic plc has received U.S. FDA approval for Pipeline™ Flex Embolization Device with Shield Technology™. Background An estimated 500,000 people throughout the … continue reading “U.S. FDA Approves Pipeline™ Flex Embolization Device with Shield Technology™”
Fourth FDA Breakthrough Device Award for Sirolimus DEB
The FDA has awarded MedAlliance breakthrough status for its SELUTION SLR™ sustained limus release Drug Eluting Balloon (DEB) catheter, in … continue reading “Fourth FDA Breakthrough Device Award for Sirolimus DEB”
FDA Approves Expanded Labeling of Medtronic MRI Leads
Medtronic plc says it has received approval from the U.S. FDA for expanded MRI labeling of its InterStim™ II and InterStim™ … continue reading “FDA Approves Expanded Labeling of Medtronic MRI Leads”
FDA Clears Vetex ReVene Thrombectomy Catheter
Vetex Medical Ltd. tells us that the U.S. FDA 510(k) has cleared its the ReVene® Thrombectomy Catheter. Vetex expects to make the … continue reading “FDA Clears Vetex ReVene Thrombectomy Catheter”
Q‑Flow™ 2021 Targets Perfect Surgical Site Illumination
Surgical light technologist Merivaara has revealed its new improved Q-Flow™ 2021 offering. The system has been reformed in key features … continue reading “Q‑Flow™ 2021 Targets Perfect Surgical Site Illumination”
CE Mark for Robotic Cancer Detection Probe
Now with CE mark approval, Lightpoint Medical’s SENSEI® becomes the first robotic gamma probe to be commercially available to European … continue reading “CE Mark for Robotic Cancer Detection Probe”
CMS Code for Intersect ENT PROPEL® Sinus Implant
Ear, nose and throat (ENT) specialist Intersect ENT®, Inc. tells us about a new Centers for Medicare and Medicaid Services (CMS) … continue reading “CMS Code for Intersect ENT PROPEL® Sinus Implant”
De Novo FDA Nod for Miach ACL Healing Implant
Miach Orthopaedics, Inc., has announced that the U.S. FDA has granted the company’s De Novo Request for the Bridge-Enhanced® ACL Repair (BEAR®) Implant.
FDA Clearances Enhance Mazor™ Robotic Guidance System
The U.S. FDA has cleared the use of navigated interbody and Midas Rex™ high speed drills with the Mazor™ Robotic Guidance System earlier than originally anticipated
MedShape Launches DynaNail Mini® for Medial Column Fusion
Additional size offerings expands company’s portfolio of limb salvage surgical solutions. Background MedShape, Inc., the industry leader in orthopedic devices … continue reading “MedShape Launches DynaNail Mini® for Medial Column Fusion”
FDA OKs ReCor Paradise™ Ultrasound Renal Denervation
ReCor Medical’s Paradise™ Ultrasound Renal Denervation System has received Breakthrough Device Designation from the U.S. FDA for the treatment of patients with uncontrolled hypertension who are inadequately responsive to anti-hypertensive medications.
Carpediem™ First Cardio-Renal Pediatric Dialysis Launch
Medtronic plc has announced the U.S. commercial launch of the Carpediem™ Cardio-Renal Pediatric Dialysis Emergency Machine, the first and only … continue reading “Carpediem™ First Cardio-Renal Pediatric Dialysis Launch”
CE Mark for ORTHO Optix™ Reader
CE mark for ORTHO Optix™ Reader means lower volume transfusion labs in Europe will now be able to offer results … continue reading “CE Mark for ORTHO Optix™ Reader”
Penumbra’s Indigo® Cleared for Pulmonary Embolism
Penumbra, Inc. has announced U.S. FDA 510(k) clearance for expanded indication of the Indigo® Aspiration System, Lightning™ 12. Background Pulmonary embolism, … continue reading “Penumbra’s Indigo® Cleared for Pulmonary Embolism”